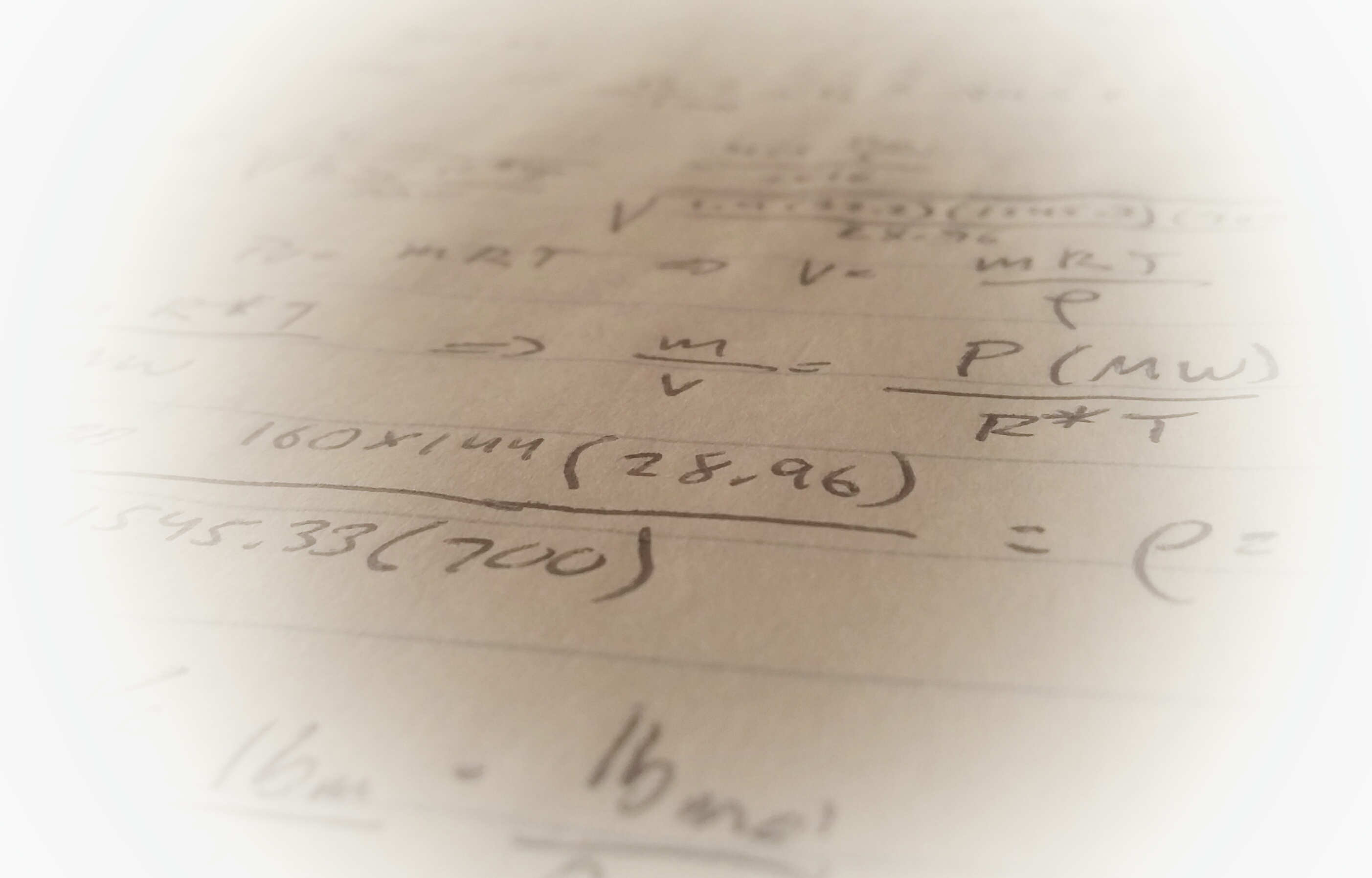
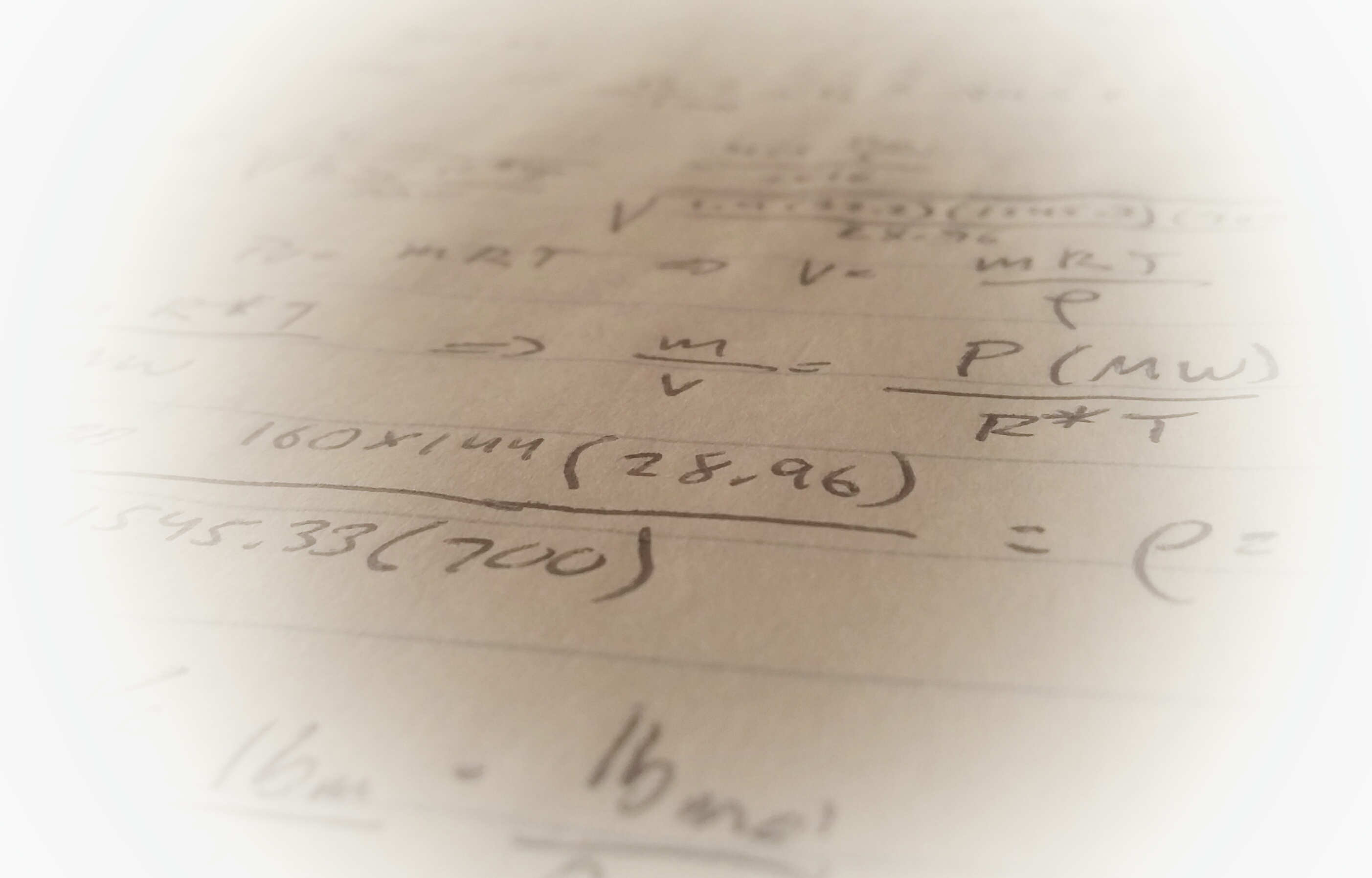
Sensible heat change is a change in temperature and no change in humidity ratio. I.e. a sensible cooling process would be a process where air is cooled but the amount of water vapor in the air doesn’t change (no condensation). The most common example of this process is simple air conditioning. Let’s look at a very simple example to illustrate how psychrometric processes are mapped on the psychrometric chart:
Air enters an air conditioner at 88°F with a relative humidity of φ=70%. It moves across the AC units cooling coils and is sensibly cooled to a temperature of 78 °F. What is the relative humidity of this air?
This process can be entirely solved using the psychrometric chart. Start with your known air characteristics:
Find the intersection of these two characteristics on the chart:
The cooling done is sensible so the humidity ratio is constant, therefore the process moves along the humidity ratio line until it intersects with the dry bulb temperature in state 2 (78 °F):
From here we can see the relative humidity is almost to the saturation line at about 98%.
PE exam strategy: This problem specifically mentioned sensible heating but what if it hadn’t? The fact that the problem statement never mentioned anything about steam flowrate or condensation rate might hint at this being sensible cooling only. Non sensible (i.e. latent) heating and cooling would have an associated transfer of liquid into (humidification) or out of (dehumidification) your air mixture. Sometimes what’s NOT said in a problem statement can help you find a path forward!
Latent heat is essentially the heat associated with a change in state. In the case of Psychrometric/HVAC applications this change in state is typically water vapor changing to liquid (dehumidification) or liquid water changing to vapor (humidification). These changes in state do not change the actual sensible temperature. Think of it like an ice cube melting; heat energy is required to melt a cube of ice. During this process, both the ice and the corresponding liquid after the state change are both at the same temperature of 0°F. The sensible heat change was zero, but the total energy between the two states (latent plus sensible heat) is non zero because you know intuitively heat must’ve been transferred in order to melt the ice. In terms of the psychrometric chart, this corresponds with processes along a constant dry bulb temperature lines:
For any questions or corrections on this material drop us a line at info@passthepeexam.com