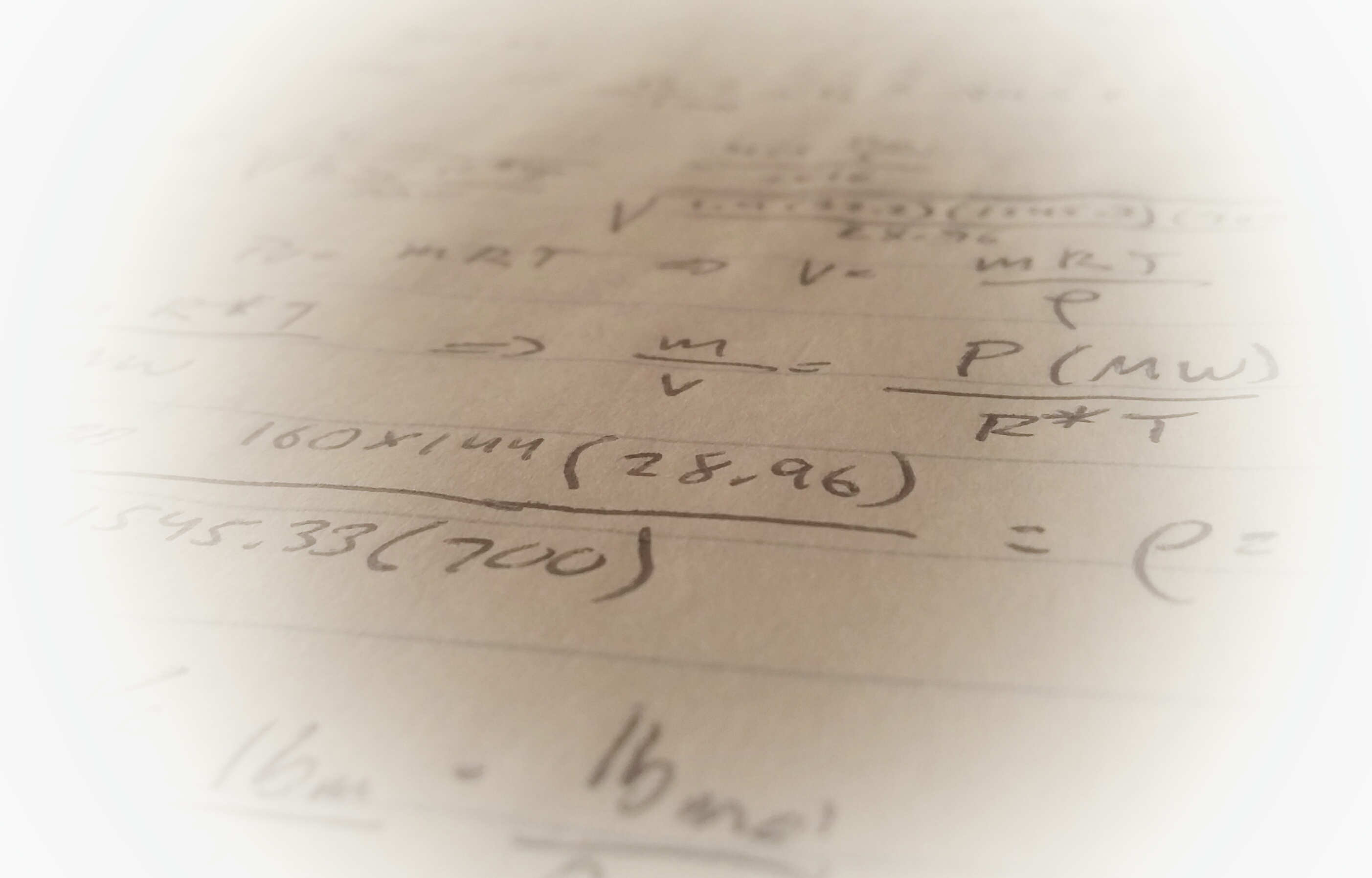
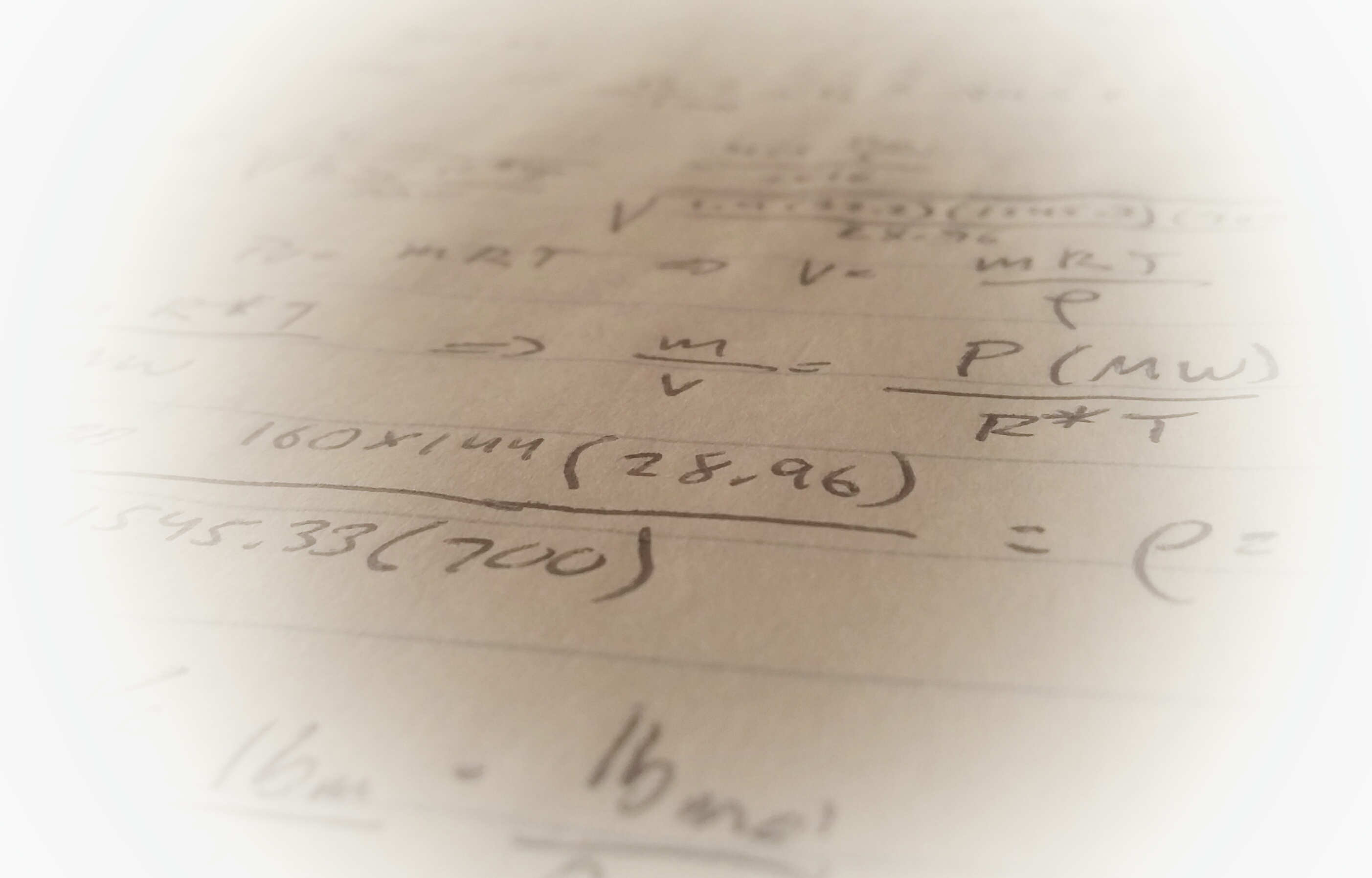
120 scfm of air flows in a duct at a dry bulb temperature of 89F with a relative humidity of φ=0.70. A condensate drain collects 0.3 gal/hr of condensate from outside the cooling coils. The air leaving the duct after passing over the cooling coils is at a dry bulb temperature of 70F.
How many tons of cooling capacity is required from the cooling medium to accommodate this process?
What this question is really asking is how much heat energy is transferred from the air to the cooling medium (i.e. cooling capacity). To determine this, we can calculate the enthalpy change of the air before and after is passes the cooling coils
The problem statement gives you the amount of condensate leaving the air stream so this process has to be a combination of cooling and dehumidification. To determine the amount of dehumidification the process sees we need to convert the given condensate of 0.3 gal/hr into the terms given on the psychrometric chart of lbs of moisture per lbs of dry air.
Using your notes (free equations sheet here), convert 0.3 gal/hr of water to lbs/hr:
This calculation tells us we’re losing 2.5 lbs per hour from our cooling process. This translates into a difference in humidity ratio (W) of:
To complete this calculation we need the mass of dry air moving through the duct. We are given the volumetric flow (acfm) of air in the duct so we can use the specific volume to calculate mass.
From the psychrometric chart, the specific volume of air at 89F and φ=0.70 is ν=14.25 cu ft/lb-dry air. A simple calculation using the given flow parameter and this specific volume give the weight of dry air:
Plugging this back into our humidity ratio calculation:
Now we have two distinct properties at both air states; Tdb= 89F and φ=0.70 at state 1 before the cooling coils and Tdb=70F and humidity ratio W=0.00495 lbv/lba . From these two points we can follow the enthalpy lines to determine the air enthalpy at both points. We can use these to map this process on the psychrometric chart:
From here we know the enthalpy of point 1 is h1=45 btu/lb-dry air and point 2 is 22 btu/lb-dry. In this case the cooling capacity required of the cooling medium is essentially how much heat energy the cooling medium is required to take out of the air or in other terms:
Note we’ve already calculated the lbs of dry air as 505.2 lb/hr so the capacity (heat/time) is:
The problem statement asks for tons of cooling capacity which is a simple conversion:
PE exam strategy: 12000btu/hr=1ton is a common conversion needed for the exam. Memorize it or keep it handy in your notes. See the free PE Exam notes on the homepage for popular conversions.
For any questions or corrections on this material drop us a line at info@passthepeexam.com